CLEANING AGENTS
In the realm of housekeeping, ensuring spaces are not only clean but also sanitized and welcoming is paramount. To achieve this, housekeeping staff relies on a variety of cleaning agents tailored to different surfaces and cleaning needs. These agents range from general-purpose cleaners to specialized solutions for tackling specific stains and surfaces. Here's a breakdown of some common types of housekeeping cleaning agents:
WATER
Referred to as the universal solvent, this is the prime agent in the cleaning process. However, though an excellent solvent, water alone is not sufficiently effective cleanser to meet the standards most hotels require. Indeed it does not wet a surface properly as its surface tension prevent it from spreading easily. For water to be effective in cleaning it must be used in conjunction with other cleaning agents such as detergents, soaps, and so on. From the prospective of cleaning there are two type of water – hard and soft. Soft water is ideal for cleaning purposes and also to make up the proper dilutions of other cleaning agents.
Hard Water and Soft Water
Water that contains more than 60 ppm (parts per million) of calcium or magnesium is called hard water. When the mineral content is in the range of 61-120 ppm, the water is said to be moderately hard and if it is exceeds 180 ppm, the water is considered very hard. When the level of dissolved calcium and or magnesium is below 60 ppm it is said to be soft water.
Temporary hardness: This is caused by carbonates of calcium and magnesium being dissolved in water. Temporary hardness is so called because it can be removed by simply heating the water to a temperature above 72۫ C.
Permanent hardness: This is caused by sulphates and chlorides of calcium and magnesium dissolved in water. It cannot be removed by boiling and requires chemical treatment to render the water ‘soft’.
Effects of hard water
Calcium and magnesium salts dissolved in water inhibit lather formation from soaps and detergents, so that much more detergent will have to be added to precipitate out the calcium and magnesium before cleaning can occur. This process causes a lot of scum to be formed, which may further soil the surface. When hard water is used for laundering, for instantce, it causes premature ageing of fabrics due to constant friction with deposits from hard water. The fabric also becomes coarse and uncomfortable to wear. Hard water also causes scale and fur to be deposited in boilers, pipes, and various appliances. Iron and sulphur salts causes discolouration... sulphur also causes a rotten egg odour. Dissolved phosphates, on the other hand can actually enhance the cleaning power of some detergents!
Methods of softening water
Water which has hardness greater than 50 ppm needs to be softened. Temporary hardness can be removed by boiling the water above 72۫C. In reaction that takes place at these temperatures, dissolved bicarbonates precipitate out as scum or fur. These could be removed by filtration before using the water for cleaning.
Ca (HCO3)2→ CaCO3↓+CO2↑+H2O
In this case of magnesium bicarbonates, the resultant carbonate further decomposes into magnesium hydroxide.
Mg (HCO3)2 →MgCO3 + CO2 ↑+ H2O
MgCO3 + H2O →Mg (OH)2 ↓ + CO2 ↑
The most practical way of removing hardness, however, is to treat it chemically. This is done in one of the following ways:
Alkali method: The alkali calcium hydroxide is used to remove the hardness from water in this method.
Lime soda method: In this method, sodium carbonate and calcium hydroxide are both used to remove the hardness.
Addition of sequestering/chelating agents: Sequestering agents are organic or inorganic compounds that react with metallic ions and form a complex. These metallic ions will be present in the water, but will be unable to react with soaps or detergents as they are held in the complex formation. Thus the water is rendered soft. The most commonly used sequestering agents are EDTA (ethylene diamine tetraacetic acid), NTA (nitrilo triacetic acid) and sodium hexametaphosphate.
Ion-exchange method or zeolite process: Zeolites are hydrated silicates of sodium and aluminium. Hard water is made to percolate through the zeolite. In the chemical ion-exchange reaction takes place, any hardness is almost totally removed. Ion –exchange units are available as attachments that can be fitted into the plumbing system at the point where the water supply enters the hotel.
Organic base-exchange method
Organic base exchanger are synthetic resins containing the sulphonic and carboxylic acid groups. When hard water is passed through the resins, the acids react with the calcium and magnesium salts to produce products which are non reactive.
DETERGENTS
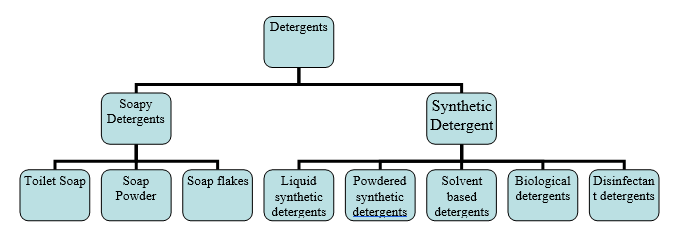
These are cleaning agents that when used in conjunction with water, loosen and remove dirt and then hold it in suspension so that the dirt is not re-deposited on the cleaned surface. They can be two types-soapy detergents and synthetic detergents.
Composition and action of detergents
All detergents are composed of three parts.
Active ingredients: In soapy detergents, the active ingredients is obtained from natural oils and fats. These are composed of long fatty –acid chains. The fatty acids commonly found in nature are the palmitic, stearic, oleic, and linoleic acids. Theses fatty acids occurs in nature as triglycerides. The active ingredients in synthetic detergents are the surface active agents or surfactants obtained from petrochemicals.
Builders: These give bulk to the detergent. A builder is a compound that has no surface-active properties but increase the efficiency of the detergent. They are added to facilitate better handling and dilution. In case of liquid detergents, the diluent can be water; in the case of powders. Sodium sulphate is used builders can be inorganic or organic.
Additives: Added to the detergent, these may be bleaching agent, blueing agents fluorescent brighteners, enzymes, and so on. Optical brighteners or fluorescent whiteners help to counteract the yellowing of fabrics that occurs with age. They are compounds that absorb ultraviolet light and reflect it back as blue light, creating an illusion of whiteness. Photo-activated bleaches on the other hand have an action that is chemical and not physical: They convert oxygen to the nascent form when activated by sunlight. Chelating agents are compound capable of binding the mineral salts that make water hard. EDTA (ethylene diamine tetramine penta acetic acid) is used to chelate iron salts. Zeolites are also being used in some detergents. hydrothropes help, when due to the presence of inorganic salts, the solubility of the liquid detergents decreases. Hydrothropes help to keep all the materials in solution. Enzymes such as proteases, lipases and amylases are incorporated into detergents to attack stains of different kinds. Advanced research as lead to the development of enzymes that are stable upto temperature of 60۫C and a pH of 10.5-11. their action is very slow and therefore they require a soaking time of 30 minutes or so. Perfumes are added to cover up the unpleasant smell of synthetic detergents and make the product more attractive. Ground pumice is added to detergents to create a coarse texture so that stubborn dirt may be removed due to friction.
How detergents work
It is the surface-active agents or surfactants in the detergents that are responsible for the three basic properties of detergents. Each molecule of the surfactant has hydrophilic (‘water –loving’) head and a hydrophobic (‘water-hating’), oleophilic (‘grease-loving’) tail. In other words, the hydrophilic head is attracted to water, whereas the hrdrophobic tail is attracted by grease and repelled by water. When the detergents is added to water, the following action takes place:
Wetting action: the detergents lower the surface tension of the water. The surfactant molecules tend to arrange themselves at the water-air interface. The hydrophobic tails of the surfactant molecules are repelled by water, creating a pull in the opposite direction to that of the inward pull of the water molecules.
Emulsifying action: The hydrophobic tails of the surfactant molecules are also oleophilic in nature, that is, they are attracted to grease. The thus penetrate the grease and lift it off the fabric surface. The dirt also gets lifted away as it is entrapped in the grease.
Suspending action: Since the grease molecules are entrapped by the surfactant molecules, their contact with other surface is prevented. The grease ( with the embedded dirt) is thus held in a stable emulsion in the water. This is also partly due to the fact that the hydrophilic heads at the other ends from the grease molecules are attracted to water. Most of the surfactant now carry a mild charge, that is they ionize and repel each other. This also aid in the suspending of the detergents.
Types of detergents
Soapy detergents/soaps: These are obtained when fat/oil is treated with an alkali. Soaps are effective only in soft water; in hard water they form a scum that is difficult to rinse away.
Toilet soaps: They are used in different kinds of packaging for guestrooms and cloakrooms. They contain perfumes, dyestuffs, and antioxidants such as vitamin E. they do not contain builders.
Soap powders: they dissolve rapidly in water and lather well, and comprise upto 40 per cent of builders.
Soap flakes: The simplest of all detergents, they dissolve easily and are used for delicate fabrics washed at lower temperatures.
Synthetic detergents: These are soap free and have replaced the use of soaps in many cleaning processes. They are not affected by hard water and have good suspending powers. Based on their chemical nature, they may be neutral detergents (anionic, non-ionic, cationic, or amphoteric) or alkaline detergents as we have seen above.
Liquid synthetic detergents: These are heavy duty detergents suitable for heavily soiled surface and fabrics. They contain 20 per cent anionic surfactants, 2 per cent non-ionic surfactant about 33 per cent alkaline builders, 9 per cent bleach, 20 per cent fillers, SCMC (sodium carboxymethyl cellulose), brighteners, and 15 per cent water.
Solvent based detergents: These contain water-miscible solvent. Their pH is around 12 and they are used for stripping spirit-based wax floor polishes.
Biological detergents: These are powered detergents to which enzymes have been added. They are used for removing organic stains at a temperature of 40-50۫C.
Disinfecting detergents/sanitizers: These are based on cationic surfactants, mainly ‘quats’ (quaternary ammonium compounds). They good germicidal and antistatic properties. They are available as cleaning gels, air fresheners, and fabric conditioners. They may be used on floors, walls, equipment, and areas that come into contact with food.
ABRASIVES
These are substance or chemicals that depend on their rubbing or scratching action to clean dirt and grit from hard surfaces.
Types of abrasives
Fine abrasive: These include precipitated whiting (filtered chalk) and jeweler’s rouge (a pink oxide of iron) used for shining silver. They are also constituents of commercial silver polishes.
Medium abrasives: These includes rotten stone, salt, scouring powder, and scouring paste. Scouring powder are made up of fine particles of pumice mixed with a soap/detergent, an alkali, and bleach.
Hard /coarse abrasives: These includes bath bricks, sand paper, pumice, steel wool, and emery paper.
Glass paper, calcite, sand paper, fine ash, emery powder and paper, jeweller’s rouge, powdered pumice, precipitate whiting (filtered chalk), feldspar, ground limestone, sand, carborundum, steel wool, and nylon scourers are some commonly used abrasives. Abrasives are usually not used alone in cleaning agents. For example, a cream or paste meant for cleaning utensils contains about 80 per cent of finely ground limestone, along with other substance such as bleaches, anionic surfactant, alkaline builders, and perfumes.
REAGENTS
These bring about cleaning by a chemical reaction requiring a distinctly low or high pH. They thus include acids and alkalis that aid in the cleaning process. To understand the action of acids and alkalis one must have knowledge of the term pH. pH is a measurement of the level of acid or alkali in a solution or substance. in the pH range of 0 to 14 a reading below 7 shows an acid and one above 7 shows an alkali.
Types of reagents:
Acids: Acid used as cleaning agents may vary from mild acid with a pH of 3 to strong acids with a pH of 1. Mildly acidic substance used commonly in cleaning includes lime, vinegar, tamarind, and buttermilk. Acids nay be used in solution alone or may be part of some special formulations, as in toilet cleaners.
Alkalis: These are used as cleaning agents in the form of liquids and powders. They are particularly useful in the laundry. Very strong alkalis should be used with utmost caution as they are corrosive and toxic. These are called as caustic alkalis. Many alkalis acts as bleaches. Caustic soda based cleaning agents are used to clear blocked drains and to clean ovens and other industrial equipment. Ammonia is a strong grease emulsifier and should also be carefully used as emits strong fumes. It is also added to abrasive formulation. Toilet cleaners to which bleach has been added are effective. It should be kept in mind that sodium chlorite bleach should never be used with an acidic toilet cleaners, however, as it will release toxic gas.
ORGANIC SOLVENTS
Grease is soluble in organic solvents such as carbon tetrachloride, acetone, turpentine, and methylated spirit. Thus these organic solvent are used extensively in the removal of grease, dry-cleaning of fabrics, and stain removal. Solvents are also useful in cleaning surfaces and are therefore ideal for cleaning glass surfaces such as mirrors and windows. Organic solvent should be handled with care as they are harmful to the skin, flammable, and poisonous.
DISINFECTANT AND BLEACHES
Disinfectant aid in the cleaning process by bringing about varying ranges of microbial control. The term ‘disinfectant’ is now used as a general term that covers all kinds of agents that bring about germ control. Most disinfectants have a strong smell and therefore should be used only in recommended amounts in areas where germ control is required.
Types of disinfectants
Phenols: These are hydroxyl derivatives of the aromatic hydrocarbon benzene. They are used in dilute or high concentration to disinfect surfaces in hospitals especially. In hotels diluted phenols are used with their sharp smell masked by other additives.
Halogens: The elements chlorine and iodine may be used as disinfectants. Chlorine is used both as bleach and as disinfectant on many surfaces. Iodine is not often used to disinfect surfaces because it tends to leave brown stains.
Quaternary ammonium compounds (‘quats’): These are cationic surfactants useful as bacteriocides.
Natural pine oils: Pine oils are obtained from oine trees. They are germicidal to some extent, but are mainly added to cleaning formulations for their pleasant smell.
GLASS CLEANERS
These are composed of an organic, water miscible solvent such as isopropyl alcohol and an alkaline detergent. Some glass cleaners also contain a fine, mild abrasive. Most glass cleaners are available as sprays or liquids. They are sprayed directly onto windows, mirrors, and other glass surfaces or applied with a soft cloth and rubbed off using a soft, lint free duster. A glass cloth is ideal for the purpose. Soft water to which some methylated spirit or vinegar is added is an expensive glass cleaner that can be readily made in the housekeeping department.
DEODORIZERS:
Deodorizer aid in the cleaning process by counteracting stale odour and sometimes also introducing a fragrance to mask them. They are used in restrooms, guestrooms, guest bathrooms, cloakrooms, and public areas such as lobbies. Some deodorizers leave no trace of a perfume cover-up. They are usually available as aerosol sprays, liquids, powders, and crystalline blocks. The crystalline blocks are effervescent and manufactured using the principle of time released aromatic chemicals. Naphthalene balls also serve as effective deodorizers. If through cleaning and good ventilation are provided, money need not be spent on expensive deodorants.
POLISHES
These are chemicals produced a shine by providing a smooth surface from which light is reflected evenly. Polishes are primarily applied to a surface to form a hard, protective layer thus guards against finger marks, stains, and scratches. Polishes are used on metal, furniture, and flooring and are classified according to the type of surface they are used on.
Classification of polishes
Metal polishes: These remove the superficial tarnish that forms on metal surfaces due to the attack of certain compounds in the air and some foodstuffs. These polishes also eliminate any scratches on the metal. They consist of very fine abrasive, generally either precipitated whiting or jeweller’s rough. Most polishes also contain a fatty acid, a solvent and water. On buffing they remove tarnish and produce a shine, e.g. brasso and silvo.
Furniture polishes: These contain a wax or resin, a solvent, water, and a silicone. The wax or resin helps to keep the furniture surface supple. It also protects against abrasion and absorption of stains and spills. The main role of wax, however is to provide a smooth surface from which light is reflected evenly, producing an attractive sheen. The types of waxes commonly used are carnauba, beeswax, ozokerite, and paraffin wax. The solvent and water are meant to remove grease stains and water soluble stains, respectively. Silicone is used to make the polish easier to apply. It also gives an added gloss and improves resistance to moisture, heat, dust, and smears. Silicone thus give a harder and longer-lasting finish.
Paste polishes: These have a higher percentage of wax (25-30 per cent). They may or may not contain silicones. They are ideal for use on antique wood furniture. Pastes should be applied in small amounts, buffed for a long time to get the desired result, and care should be taken to remove all traces of excess polish from carved areas afterwards.
Cream polishes: These have a high percentage of solvent. They contain light coloured waxes. Creams need to be used on furniture with a gloss finish only as they gradually increase the shine after continual use on the surface. Because of the higher solvent content, they have a strong smell and should be used in a well ventilated room to let the fumes they exude dissipate completely. They are applied with a dry or damp rag and the surface polished up immediately with a duster.
Spray-on polishes: These contain about 8 per cent wax and a high amount of silicone. Sprays contain aerosol to make their application simpler. These polishes clean as well as polish, and pre-dusting of the surface is not required. They are ideal use on non-porous surfaces such as glass, chromium, plastic and varnished or gloss painted wood. They reduce the static electricity on the surface so that dust is not attracted to it readily.
DISTRIBUTION AND CONTROL OF CLEANING AGENTS
The housekeeping should implement proper systems for the methodical issuing of cleaning agents from the housekeeping stores. Stores may be issued in the following way:
Requisitioning: This system of issuing is followed in large hotels. The floor supervisor maintains a requisition slips in triplicate. A requisition slip is filled by a GRA whenever supplies are diminishing. This is signed by the floor supervisor and the book is then sent to the housekeeping stores. The storekeeper collects the requisitioned items and signs the triplicate copies of the requisition slip. The storekeeper then issues requisitioned items which are collected by a porter and transported to the floor in question with signed one copy of the requisition slip. The second copy is sent to the executive housekeeper and the third copy remains in the requisition book, which too is returned with the fresh supplies.
Full for Empty: This system of issuing is followed in smaller hotel. Empty containers of used up cleaning supplies are taken to the housekeeping stores by individual GRAs. The stores assistant then replaces the empty containers with full ones. The disadvantage in this system are that it works well only when the housekeeping Stores are open round the clock and that constant supervision is required. The topping up method is an improvement on this system of issuing.
Topping Up: The difference between this method and the earlier one is that here the GRAs approach the housekeeping stores only at a fixed time each week for getting their supplies topped up. An even better system is having the GRAs deposit their hand caddies in the housekeeping stores at the end of the shift, so that the stores assistance may replenish or top up the cleaning agents and keep them ready for the staff on the next shift.
SELECTION OF CLEANING AGENTS
The use of cleaning agent is meant to save time, effort and money. If selected well, all the three objective may be fulfilled. The following points need to be considered when selecting cleaning agents;
1. The type of soilage
2. The type of surface
3. Odour
4. Range of action or versatility
5. composition of cleaning agent
6. Ease of use, saving of effort and time
7. Toxicity or side effects
8. shelf Life
9. Packaging volumes and quantities
10. Cost effectiveness
www.ehospitalitystudies.com


HOTEL HOUSEKEEPING DEPARTMENT


Cleaning Agents